Abstract
The benefits and adverse effects of immunosuppressive drugs (ISDs) in patients with paraquat (PQ) poisoning have not been thoroughly assessed. This meta-analysis study aims to evaluate the effect of ISDs in patients with moderate to severe PQ poisoning. We searched PubMed, Embase, Cochrane Library, Ovid Medline, CNKI and Wanfang Data from inception to January 2019. The Mantel-Haenszel method with a random-effects model was used to calculate the pooled relative risks (RRs) and 95% Confidence Intervals (CIs) as described by DerSimonian and Laird. An L’Abbé plot was drawn to explore the relationship between the degree of poisoning and mortality. Four randomized controlled trials, two prospective and seven retrospective studies were identified. ISDs were significantly associated with reduced mortality (RR 0.76; 95% CI, 0.58-0.99) and the incidence rate of multiple-organ dysfunction syndrome (MODS) (RR 0.63; 95% CI, 0.48-0.83) in patients with moderate to severe PQ poisoning. They were not associated with an increased incidence rate of hepatitis and reduced incidence rate of acute renal failure and hypoxia. The L’Abbé plot results showed a slight increase in mortality rate in the ISD group with increased mortality in the placebo group. This indicates a possible advantage of ISDs in most of the patients with severe PQ poisoning. These findings suggest that ISDs may reduce the mortality and incidence rate of MODS in moderate to severe PQ poisoning patients, and severe PQ poisoning patients might benefit more from ISDs.
INTRODUCTION
Paraquat (PQ) is a highly efficient and non-selective contact herbicide. It has been widely used in many countries since the 1960s because of its strong activity against weeds and rapid deactivation upon contact with the soil. However, it is highly toxic to humans, and there is no specific antidote or effective treatment (Gawarammana and Buckley, 2011; Sun and Lee, 2013). Acute PQ poisoning has occurred more frequently in recent years due to increasing amounts of PQ usage and it has highly fatal side effects (Gawarammana and Buckley, 2011). This has become a major public health problem associated with high mortality rates (> 50%) in developing countries (Chinese College of Emergency Physicians, 2013). To date, there is no specific antidote for acute PQ poisoning, with the mortality rate (50-70%) high in patients that suffer PQ poisoning (Chinese College of Emergency Physicians, 2013; Xu et al., 2017).
Traditional treatment for PQ poisoning includes emetic, gastric lavage, catharsis, diuresis, and other symptomatic treatments (Chinese College of Emergency Physicians, 2013). To some extent, these can clean PQ utility in vivo and vitro, and obviate exposure to PQ. However, these procedures have been proven to be less effective in PQ poisoning.
Apart from its high mortality rate, PQ can also cause severe multiple-organ failure of the pancreas, kidneys, liver, lungs, adrenal glands, and central nervous system (Fortenberry et al., 2016; Gawarammana and Buckley, 2011; Li et al., 2015). The mechanism of PQ-induced organ injury is thought to be the production of reactive oxygen species by enzymatic one-electron reduction of PQ, followed by one-electron transfer to dioxygen with the generation of the superoxide anion. Various reactive oxygen species (O2-, H2O2, and OH-) produced from PQ activate the human immune system and induce a variety of inflammatory cytokines (Dinis-Oliveira et al., 2008; Gawarammana and Buckley, 2011; Sun and Lee, 2013).
Recent studies have reported that treatment combined with immunosuppressive drugs (ISDs) may be beneficial to patients with moderate to severe PQ poisoning (Afzali and Gholyaf, 2008; Chen, 2014; Ge et al., 2014; He et al., 2015; Hsieh et al., 2013; Jian et al., 2008; Lin et al., 1996, 1999, 2006, 2011; Vieira et al., 1997; Wang et al., 2008; Wu et al., 2014; Xu et al., 2012). However, there are still disagreements between the results of other related studies (Eddleston et al., 2003; Gawarammana et al., 2018; Perriëns et al., 1992), and not enough clinical evidence can prove these results. The study by Perriëns et al. (1992) shows that it is unlikely to improve the prognosis of PQ poisoning and Gawarammana et al. (2018) found no evidence that high dose immunosuppression improves survival in PQ poisoning patients. Hence, we performed this meta-analysis to explore the effect of ISDs in patients with moderate to severe PQ poisoning.
MATERIALS AND METHODS
Literature search
A systematic search of PubMed, Embase, Cochrane Library, Ovid Medline, CNKI and Wanfang Data, from March 1968 to January 2019 was performed to identify all published studies assessing immunosuppressive therapy for patients with moderate to severe PQ poisoning. The search strategy for PubMed was as follows: (((PQ) OR gramoxone)) AND ((((((((((Glucocorticoid) OR Methylprednisolone) OR Methylprednisone) OR metacortandracin) OR metacortandralone) OR Cortisone) OR Hydrocortisone) OR Dexamethasone) OR Betamethasone) OR cyclophosphamide). All relevant reports and reviews were hand searched for additional eligible studies. Further information was sought by correspondence with the authors in cases of data unavailability.
Study selection
Included studies: (1) Oral PQ poisoning; (2) Patients over the age of 18 with PQ poisoning; (3) Patients in the experimental group received immunosuppressive drugs (Glucocorticoid or Methylprednisolone or Methylprednisone or metacortandracin or metacortandralone or Cortisone or Hydrocortisone or Dexamethasone or Betamethasone or cyclophosphamide); (4) One or more efficacy outcomes were reported, including mortality, incidence rate of MODS, acute renal failure (ARF), hepatitis, and hypoxia.
Excluded studies: (1) Case reports; (2) With no control group; (3) No outcomes were provided relative to the purpose of our studies.
Data extraction
Two researchers collected the characteristics of the trials respectively (author, year, study design, sample size, number of ISD group case, number of control group case, population characteristics, use of plasma PQ tests, use of urine PQ tests, application of drugs in the ISD group and control group, and follow-up time), characteristics of the enrolled patients (age, gender, time elapsed to emergency room or hospital, time elapsed from ingestion to the beginning of hemoperfusion), and outcomes (mortality, incidence rate of MODS, ARF, hepatitis, and hypoxia). The primary endpoint was mortality. Secondary endpoints were the incidence of MODS, ARF, hepatitis, and hypoxia. As recommended by the Cochrane Collaboration (Margulis et al., 2014; Panic et al., 2013; Welch et al., 2016; Yoshii et al., 2009), domains of bias of the included studies for efficacy results were reviewed.
Statistical analysis
Mantel-Haenszel random-effect meta-analyses were performed for the RCTs, prospective studies, and retrospective studies (Mantel and Haenszel, 1959). Risk ratio (RR) and 95% confidence intervals (CIs) were used as common measures of association between PQ poisoning and outcomes (mortality, incidence of MODS, ARF, hepatitis, and hypoxia) across the studies. An L’Abbé plot was drawn to explore the relationship between the degree of poisoning and mortality (Ho and Tan, 2011). Heterogeneity across the trials was assessed using a standard chi-squared test with a significance being set at P < 0.10. Heterogeneity was also assessed by means of I2 statistics with significance being set at I2 > 50%. The random-effects model was used for statistical analyses as described by DerSimonian and Laird (DerSimonian and Laird, 2015). We further conducted subgroup analyses (whether glucocorticoids were used in the ISD group, a dose of cyclophosphamide (CP) <15 mg/kg/day, and period of CP ≤ 2 days) to explore possible explanations for heterogeneity. Statistical analysis was performed using Review Manager (version 5.3).
RESULTS
Characteristics of the studies
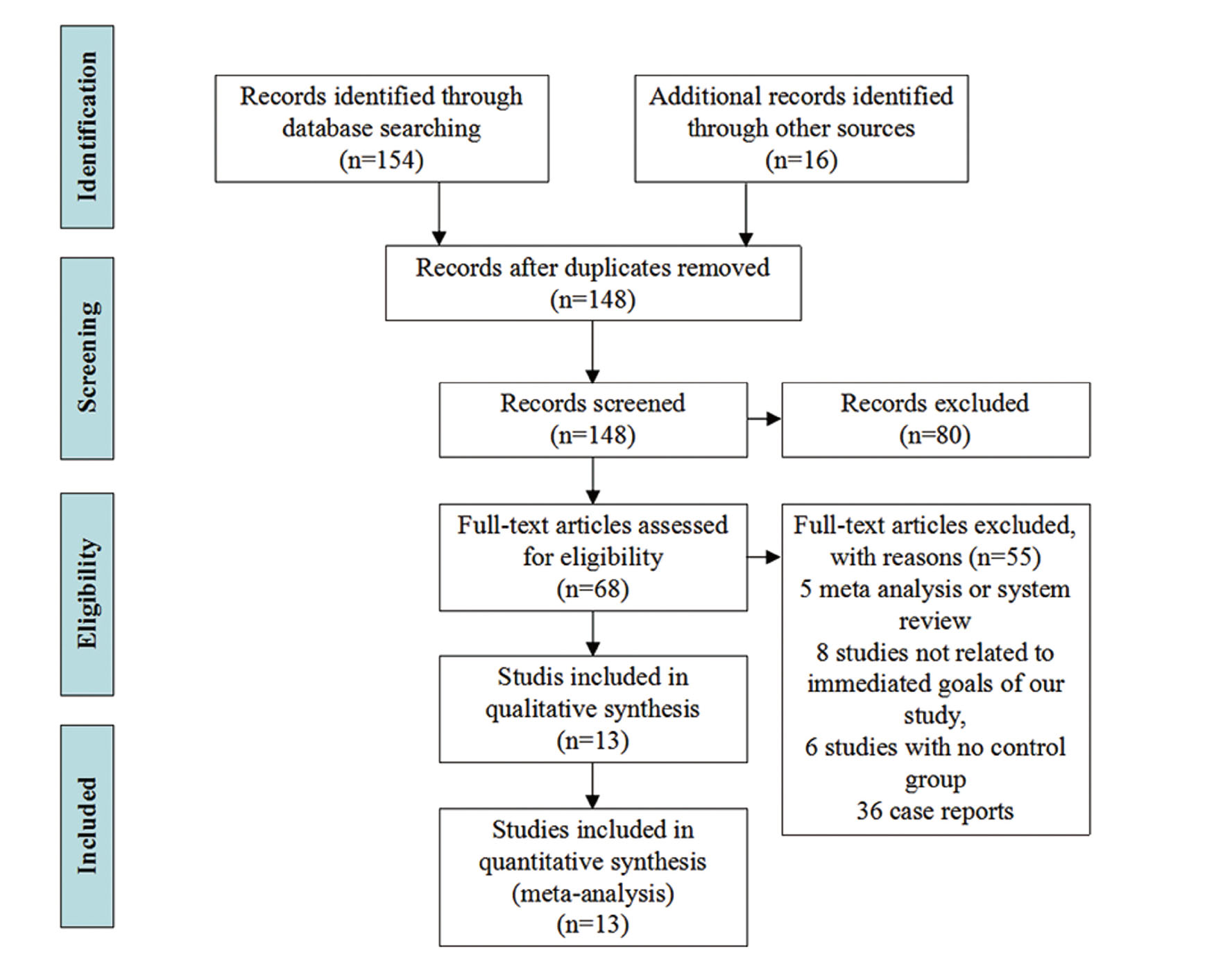
The initial search yielded 170 citations, which included 155 studies in English and 15 in Chinese. Four RCTs (Afzali and Gholyaf, 2008; Chen, 2014; Gawarammana et al., 2018; Lin et al., 2006) with 392 patients, two prospective studies (Lin et al., 1999; Perriëns et al., 1992) involving 168 patients and 7 retrospective studies (Jian et al., 2008; Lin et al., 1996, 2011; Vieira et al., 1997; Wang et al., 2008; Wu et al., 2014; Xu et al., 2012) involving 2392 patients met the criteria. Of the 157 excluded articles, 22 were repeated articles, 47 were experiments on animals, 5 were meta-analysis, 41 were not related to the immediate goals of our study, 6 were articles without control groups, and 36 were case reports, (Fig. 1).
The characteristics of the trials included in this study are listed in Table 1. All studies included in the meta-analysis involved patients with moderate to severe PQ poisoning. Of these included studies, a sodium dithionite reaction test was done on the urine samples of all the patients. In most of the studies (Afzali and Gholyaf, 2008; Chen, 2014; Gawarammana et al., 2018; Jian et al., 2008; Lin et al., 1996, 1999, 2006, 2011; Perriëns et al., 1992; Vieira et al., 1997; Wang et al., 2008; Wu et al., 2014; Xu et al., 2012), ordinarily, patients with a navy blue or a dark blue color indicated severe PQ poisoning (Koo et al., 2009; Scherrmann et al., 1987). In most of the studies (Afzali and Gholyaf, 2008; Chen, 2014; Lin et al., 1999, 2006, 2011), immunosuppressive drugs and treatment duration were CP 15 mg/kg/day for 2 days and methylprednisolone (MP) 1g/day for 3 days. Patients in nine of the studies received MP (Afzali and Gholyaf, 2008; Gawarammana et al., 2018; Jian et al., 2008; Lin et al., 1996, 1999, 2006, 2011; Wang et al., 2008; Wu et al., 2014); in six of the studies they were given dexamethasone (DEX) therapy (Gawarammana et al., 2018; Lin et al., 2006, 2011; Perriëns et al., 1992; Vieira et al., 1997; Wang et al., 2008) and Mesna and Cyclosporin A were applied in two of the studies (Afzali and Gholyaf, 2008; Xu et al., 2012). In the control group, one study used MP (Chen, 2014); one study used CP (Lin et al., 2011); and three studies took DEX (Lin et al., 2006, 1999, 2011). The characteristics of patients and the outcomes are shown in Table 2. All the studies reported a patient’s mortality, most studies gave information about age, gender, the time elapsed to the emergency room or hospital, and the time to hemoperfusion. The incidence rate of MODS, ARF, hepatitis, and hypoxia were reported in studies 4, 6, 6, 8 respectively.
Table 1. Characteristics of Included studies.
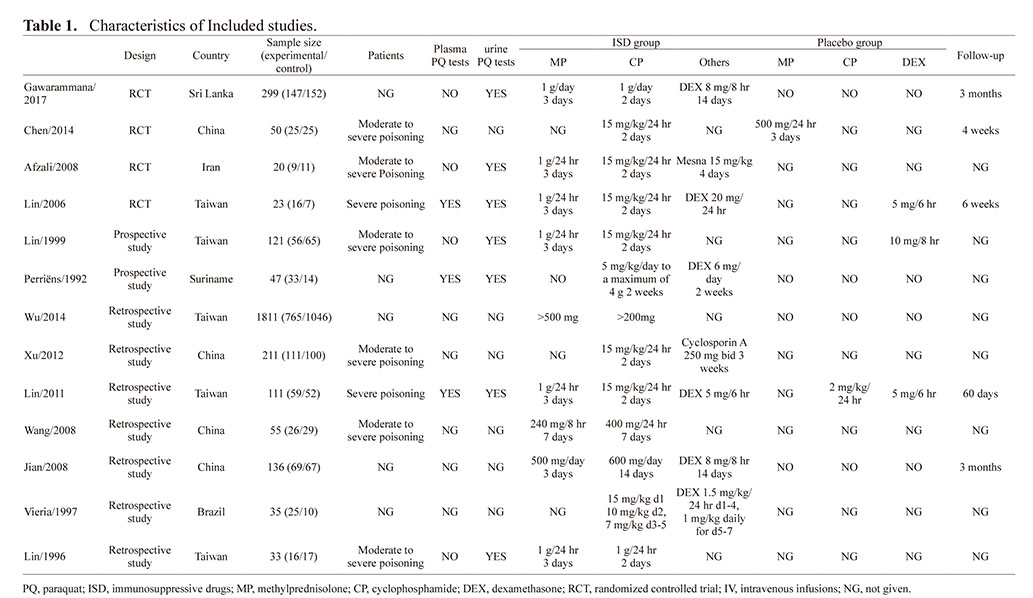
Table 2. Characteristics and outcomes of patients.
 Primary Outcome
Primary Outcome
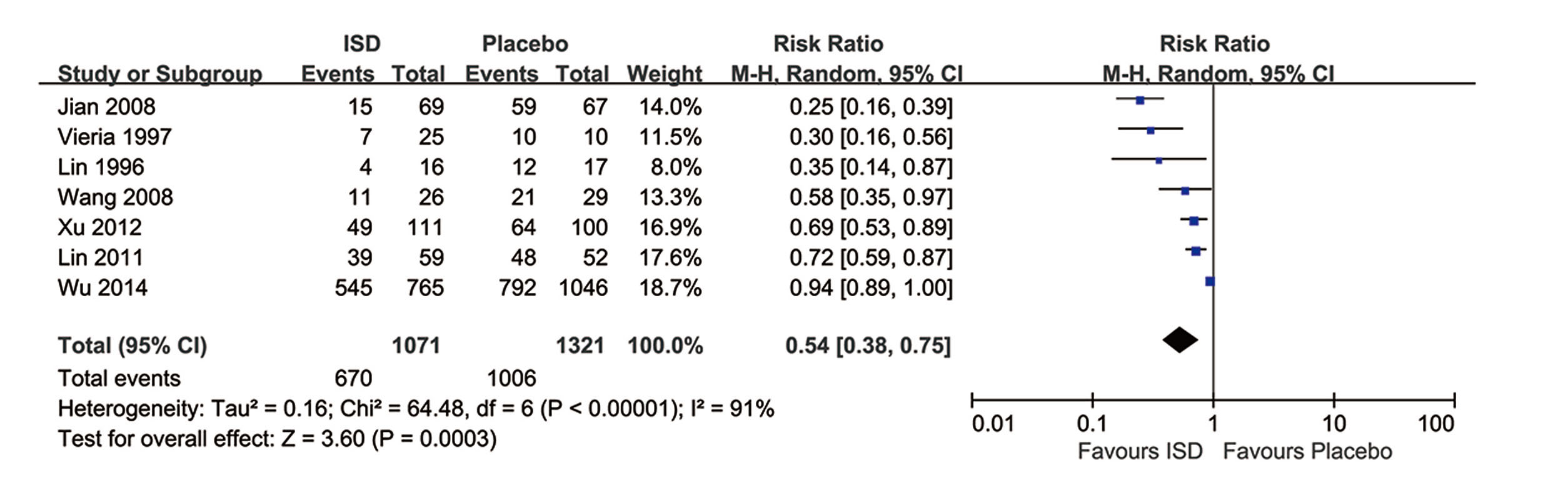
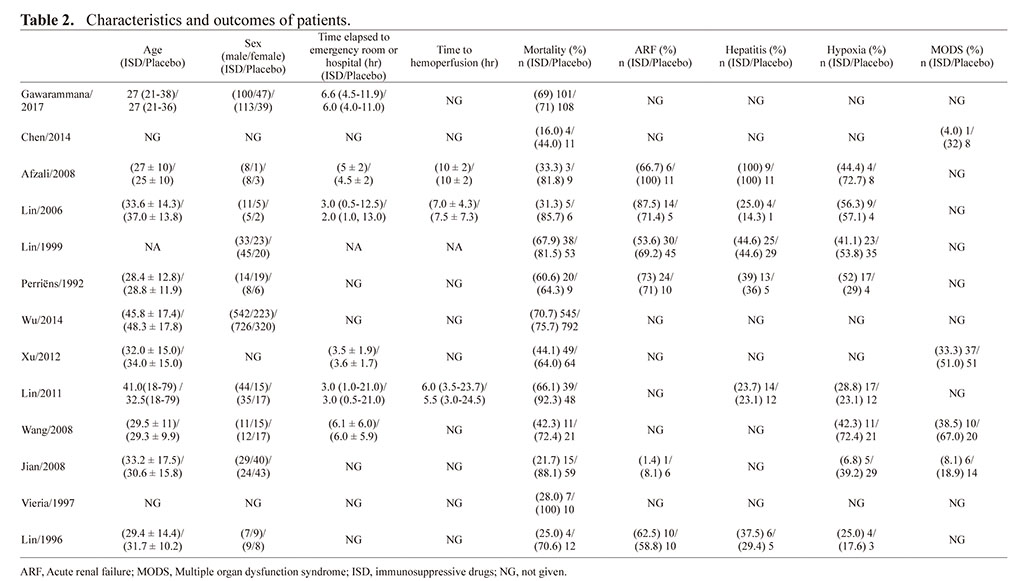
All studies were included in the analysis with mortality (Afzali and Gholyaf, 2008; Chen, 2014; Gawarammana et al., 2018; Jian et al., 2008; Lin et al., 1996, 1999, 2006, 2011; Perriëns et al., 1992; Vieira et al., 1997; Wang et al., 2008; Wu et al., 2014; Xu et al., 2012). The pooled result from RCTs and prospective studies showed that immunosuppressive treatment was significantly associated with a reduction of mortality rates (RR 0.76; 95% CI, 0.58-0.99) in patients with PQ poisoning with a moderate heterogeneity among the studies (I2 = 60%). (Fig. 2) Results of 2392 patients in 7 retrospective studies confirmed that immunosuppressive therapy was significantly associated with the reduced mortality in patients with moderate to severe PQ poisoning (RR 0.54; 95% CI, 0.38-0.75), with high heterogeneity (I2 = 91%). (Fig. 3)
The sizes of the squares denoting the point estimate in each study are proportional to the weight of the study. Diamonds represent the overall findings in each plot. For all study names, see the cited references. ISD: immunosuppressive drugs; M-H: Mantel-Haenszel.
Secondary outcome
The data were reported inconsistently and incompletely. This included incidence of MODS in four studies, incidence of ARF in six studies, incidence of hepatitis in six studies, and information of hypoxia in eight studies (Table 2).
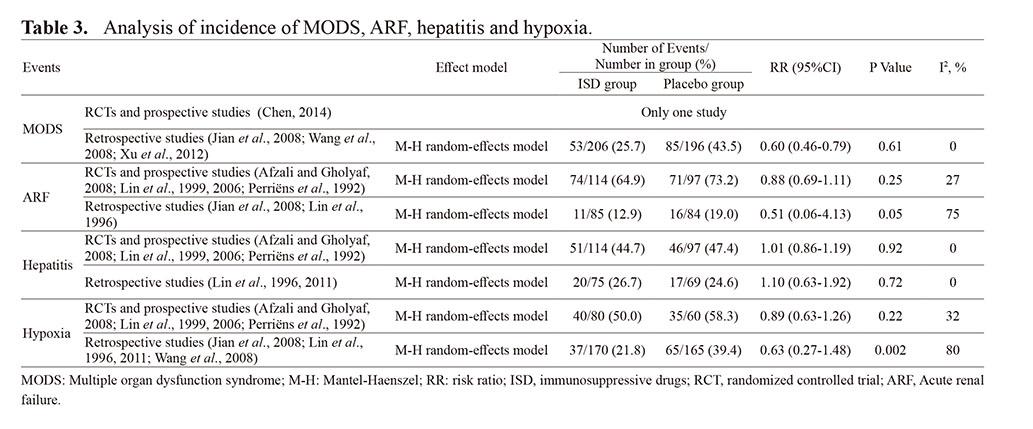
In Table 3, the pooled result from four RCTs and two prospective studies showed that immunosuppressive treatment was not associated with increased incidence rates of hepatitis (RR 1.01; 95% CI, 0.86-1.19) and reduced incidence rate of ARF (RR 0.88; 95% CI, 0.69-1.11) and hypoxia (RR 0.89; 95% CI, 0.63-1.26), patients in the ISD group demonstrated a low incidence rate of MODS (4%) vs. patients in the placebo group (32%), x2 = 6.640, P < 0.05. The analysis results of retrospective studies drew a similar conclusion compared with our findings from RCTs and prospective studies.
Table 3. Analysis of incidence of MODS, ARF, hepatitis and hypoxia.
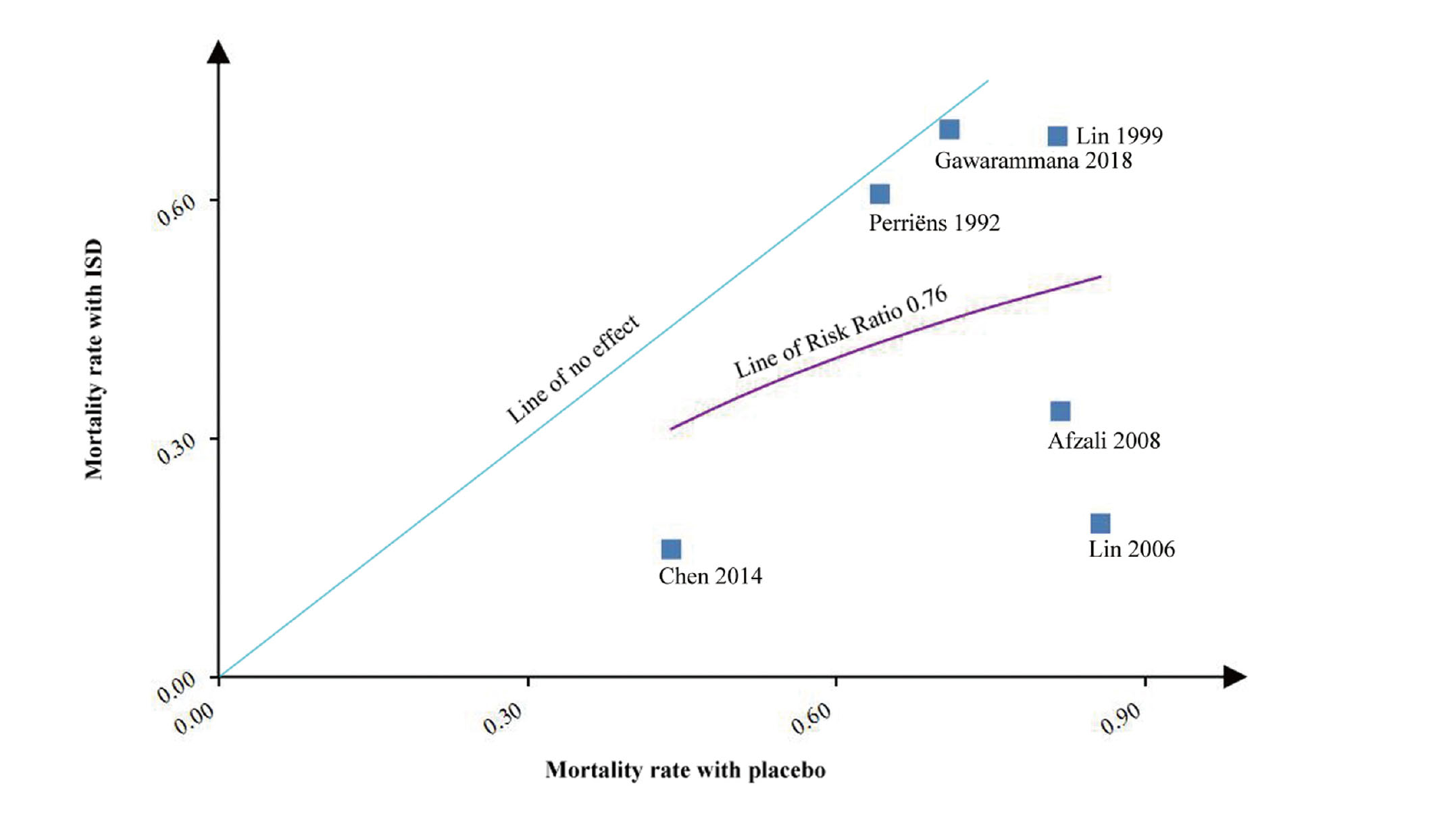
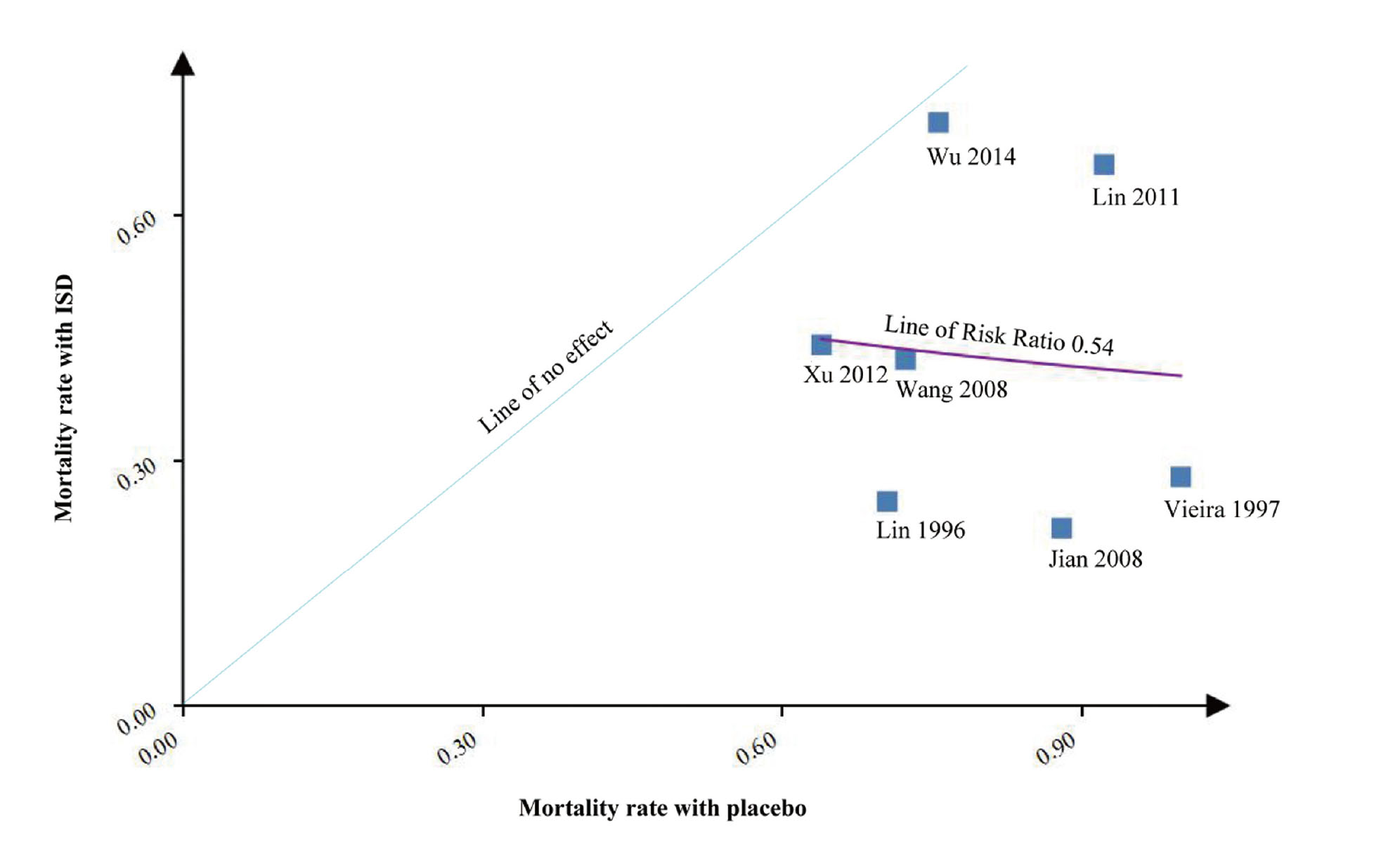
In the L’Abbé plot, compared with the increased mortality rate in the placebo group, the mortality rate increased slowly in the ISD group, which indicated a possible advantage of ISDs in most of the patients with severe PQ poisoning (Fig. 4). Figure 5 showed results from seven retrospective studies involving 2392 patients with moderate to severe PQ poisoning. We can see a reducing mortality rate in the ISD group with an increasing mortality rate in the placebo group, where immunosuppressive drugs demonstrated a possible advantage in patients with severe PQ poisoning.
The four RCTs and two prospective studies of ISDs and PQ poisoning are presented in an L’Abbé plot. With the increased mortality in the placebo group, the mortality in the ISD group increased slowly, which indicated a possible advantage of ISDs in most of the patients with severe PQ poisoning.
The seven retrospective studies of ISDs and PQ poisoning are presented in an L’Abbé plot. With the increased mortality in the placebo group, the mortality in the ISD group decreased.
Risk of Bias and Subgroup Analyses
With the evaluating tools provided by the Cochrane Collaboration (Margulis et al., 2014; Yoshii et al., 2009), risk of bias of four RCTs and two prospective studies were shown in Fig. 6. The selection, attrition and reporting biases were well-controlled in four studies. However, imbalances were reported in the degree of poisoning (Gawarammana et al., 2018; Perriëns et al., 1992), plasma PQ tests (Gawarammana et al., 2018; Perriëns et al., 1992), urine PQ tests (Chen, 2014), and follow-up time. Four studies were considered to be of high quality because the random sequence generation, allocation concealment, and blinding were not reported in the articles. Two studies were considered to be of fair quality.

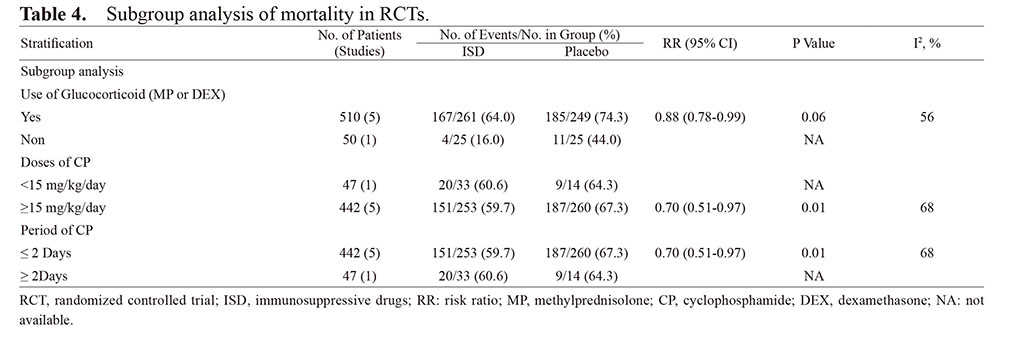
Subgroup analyses for mortality were performed to evaluate whether glucocorticoid MP or DEX was used in the ISD group, and at a dose of CP (CP < 15 mg/kg/day or CP = 15 mg/kg/day) for a period of CP (≤ 2 days) (Table 4). Subgroup analysis results showed no significant differences in the mortality rates in the group that used glucocorticoid (MP or DEX) (RR 0.88; 95% CI, 0.78-0.99), and where the dose of CP was not less than 15 mg/kg/day (RR 0.70; 95% CI, 0.51-0.97) and the period of CP was not more than 2 days (RR 0.70; 95% CI, 0.51-0.97). Results of this subgroup should be explained cautiously due to the finite sample size and potential bias that was inherent to the subgroup analysis.
Table 4. Subgroup analysis of mortality in RCTs.
DISCUSSION
Our meta-analysis identified four RCTs, two prospective studies and seven retrospective studies including 2952 patients, to investigate the effect of ISDs on mortality in patients with moderate to severe PQ poisoning. We found that ISDs were significantly associated with decreased mortality and a reduced incidence rate of MODS in PQ poisoning patients, and not associated with an increased incidence rate of ARF, hepatitis, and hypoxia.
In recent years, a large number of clinical tests and case reports indicated that ISDs are beneficial to patients with moderate to severe PQ poisoning. However, several studies showed no improvement with the treatment of ISDs. Of these studies included in our meta-analysis, four RCTs and two prospective studies assessed the effect of ISDs on the mortality of patients with moderate to severe PQ poisoning (Afzali and Gholyaf, 2008; Chen, 2014; Gawarammana et al., 2018; Lin et al., 1999, 2006; Perriëns et al., 1992). Of these six studies, four demonstrated that treatment with ISDs may be beneficial in treating patients with moderate to severe PQ poisoning (Afzali and Gholyaf, 2008; Chen, 2014; Lin et al., 1999, 2006), while two found no significant evidence that ISDs can improve the prognosis of PQ poisoning patients (Gawarammana et al., 2018; Perriëns et al., 1992).
A recent systematic review regarding PQ-induced lung fibrosis treated with ISDs and the need for a better prediction of the outcome showed no possible benefits from immunosuppressive therapy (Eddleston et al., 2003). In this systematic review (Eddleston et al., 2003), four included studies lacked a control group (Addo and Poon-King, 1986; Addo et al., 1984; Botella de Maglia and Belenguer Tarín, 2000; Garcia et al., 2000) and two studies were case reports (Chen et al., 2002; Chomchai, 2003), which may have resulted in low reliability. Our previous studies have shown that ISDs may reduce the incidence of MODS in patients with moderate to severe PQ poisoning (Gao et al., 2018). Another systematic review that assessed the effect of immunosuppressive therapy on PQ-induced lung fibrosis (Li et al., 2014) reported beneficial results with immunosuppressive therapy for patients with PQ-induced lung fibrosis, but in this study, we did not find relevant information about lung fibrosis as this was not reported in three of the included studies (Afzali and Gholyaf, 2008; Lin et al., 1999, 2006). Hence, the results of the study by Li et al. (2014) are not convincing. In the meta-analysis conducted by He et al. (2015) that was completed in 2015, three RCTs (Afzali and Gholyaf, 2008; Lin et al., 1999, 2006) and two retrospective studies (Lin et al., 1996; Vieira et al., 1997) were combined to calculate the pooled relative risks. Because the Cochrane Collaboration did not recommend merging conversion data, the results might be misleading. Though several sensitivity analyses were used to avert the possible bias, the pooled results still had a high degree of heterogeneity, which might lead to a misleading conclusion. Compared with this three system review and meta-analysis, our meta-analysis included more RCTs to make our result more reliable. To reduce the possible bias resulting from the data conversion, we separated out the RCTs from the retrospective study, to make the result more believable.
A retrospective observational study conducted on patients with herbicide poisoning admitted to the emergency ward of the tertiary care hospital in South India between January 2004-2012 by Harika Cherukuri et al. showed that poisoning with herbicides is associated with high morbidity and mortality, and treatment with N-acetylcysteine, vitamin C, vitamin E, CP, hemodialysis and hemoperfusion may be useful in the reduction of the high mortality rate (Cherukuri et al., 2014). Other studies evaluated the effectivity of the «Caribbean scheme» (CP, DEX, furosemide and vitamins B and C) drew a conclusion that it was associated with a lesser mortality rate in the subjects who ingested ≤ 45 mL of 20% PQ solution (Botella de Maglia and Belenguer Tarín, 2000. However, these two studies lacked a control group, so it was difficult to draw firm conclusions and therefore we excluded these two studies to ensure the credibility of our study. In some other observational studies, the study by Jie Gao et al. states that prolonged MP therapy after pulse treatment can reduce the mortality of moderate-to-severe PQ poisoning patients (Gao et al., 2017). Early hemoperfusion may improve the survival chances of PQ poisoning patients, as was confirmed in three studies (Gao et al., 2015; Hsu et al., 2012; Wang et al., 2017). A multivariate logistic regression analysis showed that the addition of vitamin C to the treatment was significantly associated with increased survival of the patients in the study by Moon and Chun (2011). A CP dose was assessed in Sun and Lee’s study (2013). Though these studies were not related to the immediate goals of our study, they show a likely benefit of ISDs in PQ poisoning patients. Also, it is worth mentioning that a large number of case reports suggested that immunosuppressive therapy was beneficial to patients with moderate to severe PQ poisoning. Seven cases of necrosis of the femoral head after acute PQ poisoning were reported and in two studies patients were treated with glucocorticoid and CP. This should be administered cautiously in PQ poisoning patients (Tian et al., 2010; Wang et al., 2013). Although there is no convincing evidence for toxicity from ISDs in humans, our meta-analysis indicated that ISDs may reduce the mortality and incidence of MODS in patients with PQ poisoning. To make our conclusion more convincing and reduce obvious biases, we included more clinical studies and separated out the RCTs from the retrospective studies. The association of ISDs to reduce the incidence rate of MODS was not found in any other previous meta-analysis studies. Patients’ decreased mortality rates were associated with ISDs with the reduced incidence rate of MODS maybe being one reason for this. MODS is a main aetiology of death in these patients (Afzali and Gholyaf, 2008) and is associated with a PQ-induced organ injury mechanism that involves various reactive oxygen species produced from the PQ active human immune system and induced a variety of inflammatory cytokines (Gawarammana and Buckley, 2011; Sun and Lee, 2013). ISDs can reduce the incidence rate of MODS indirectly by reducing the production of inflammatory cytokines. The possible explanation for high heterogeneity regarding the effect of ISDs on patient’s mortality was that the type of drugs used was different. Of these five studies, two used CP, MP, and DEX, two used CP and MP, one combined CP and DEX.
Our meta-analysis has limitations. Firstly, the sample size of the included studies was small. Compared with other system reviews and meta-analysis, we included the most studies in our meta-analysis, and in order to make up the disadvantages, results of the pooled data from the retrospective study were used to prove our findings on RCTs, which may support our results to some extent. Secondly, the type of drug used was different; two studies did not use MP that belongs to the ISD group and three did not use DEX, hence we conducted subgroup analysis to avoid possible bias and find more believable results. Thirdly, the severity of the illness was not consistent across the studies. Patients in four RCTs suffered moderate to severe PQ poisoning, which may be due to the measuring error in plasma or urine PQ tests, hence we have drawn an L’Abbé plot, the result showing a possible advantage of ISDs in most of the patients with severe PQ poisoning.
In conclusion, the present meta-analysis study indicated that immunosuppressive therapy may reduce the mortality and incidence rate of MODS in patients with moderate to severe PQ poisoning. Severe PQ poisoning patients may benefit more from ISDs. Our study suggests that immunosuppressive treatment was not associated with an increased incidence of hepatitis and reduced incidence of ARF, and hypoxia. More research is needed to confirm this result.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (No. 81701893), Key Scientific Research Projects of Higher Education Institutions in Henan Province (No. 20A320046), and Provincial Ministry Co-construction Project from Medical Scientific and Technological Research Program of Henan Province (No. SB201901006).
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Addo, E. and Poon-King, T. (1986): Leucocyte suppression in treatment of 72 patients with paraquat poisoning. Lancet, 1, 1117-1120.
- Addo, E., Ramdial, S. and Poon-King, T. (1984): High dosage cyclophosphamide and dexamethasone treatment of paraquat poisoning with 75% survival. West Indian Med. J., 33, 220-226.
- Afzali, S. and Gholyaf, M. (2008): The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Arch. Iran Med., 11, 387-391.
- Botella de Maglia, J. and Belenguer Tarín, J.E. (2000): [Paraquat poisoning. A study of 29 cases and evaluation of the effectiveness of the “Caribbean scheme”]. Med. Clin. (Barc.), 115, 530-533.
- Chen, G.H., Lin, J.L. and Huang, Y.K. (2002): Combined methylprednisolone and dexamethasone therapy for paraquat poisoning. Crit. Care Med., 30, 2584-2587.
- Chen, Y.P. (2014): Effect of early intensive immunosuppressive therapy on moderate to severe paraquat poisoning. Zhongguo Jiceng Yiyao, 21, 3305-3306.
- Cherukuri, H., Pramoda, K., Rohini, D., Thunga, G., Vijaynarayana, K., Sreedharan, N., Varma, M. and Pandit, V. (2014): Demographics, clinical characteristics and management of herbicide poisoning in tertiary care hospital. Toxicol. Int., 21, 209-213.
- Chinese College of Emergency Physicians. (2013): Experts consensus on diagnosis and treatment of acute paraquat poisoning (2013). Chin. Critical. Care Med., 33, 484-489.
- Chomchai, S.C. (2003): Treatment of moderate to severe paraquat poisoning with dexamethasone/cyclophosphamide combination: a case series from the toxicology consultation service at Siriraj Hospital, Bangkok, Thailand. J. Toxicol. Clin. Toxicol., 41, 520-521.
- DerSimonian, R. and Laird, N. (2015): Meta-analysis in clinical trials revisited. Contemp. Clin. Trials, 45 (Pt A), 139-145.
- Dinis-Oliveira, R.J., Duarte, J.A., Sánchez-Navarro, A., Remião, F., Bastos, M.L. and Carvalho, F. (2008): Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol., 38, 13-71.
- Eddleston, M., Wilks, M.F. and Buckley, N.A. (2003): Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM, 96, 809-824.
- Fortenberry, G.Z., Beckman, J., Schwartz, A., Prado, J.B., Graham, L.S., Higgins, S., Lackovic, M., Mulay, P., Bojes, H., Waltz, J., Mitchell, Y., Leinenkugel, K., Oriel, M.S., Evans, E. and Calvert, G.M. (2016): Magnitude and characteristics of acute paraquat- and diquat-related illnesses in the US: 1998-2013. Environ. Res., 146, 191-199.
- Gao, J., Feng, S., Wang, J., Yang, S. and Li, Y. (2017): Prolonged methylprednisolone therapy after the pulse treatment for patients with moderate-to-severe paraquat poisoning: A retrospective analysis. Medicine (Baltimore), 96, e7244.
- Gao, Y., Zhang, X., Yang, Y. and Li, W. (2015): Early haemoperfusion with continuous venovenous haemofiltration improves survival of acute paraquat-poisoned patients. J. Int. Med. Res., 43, 26-32.
- Gao, Y.X., Wang, Y.B., Wan, Y.D., Li, Y., Yuan, D., Sun, P., Sun, C.H., Che, L., Duan, G.Y., Xu, Z.G. and Sun, T.W. (2018): Immunosuppressive drugs reduce the incidence of multiple-organ dysfunction syndrome in patients with moderate to severe paraquat poisoning: meta-analysis. Zhonghua Jizhen Yixue Zazhi, 27, 923-928.
- Garcia, J., Frontado, C., Tilac, C., Rendon, C., Brewster, F., Gonzalez, A., Nazzoure, J., Flores, L., Guipe, S., Vargas, S., Medina, R. and Pernalete, N. (2000): Intoxication moderate to severe by paraquat therapy with steroid immunosuppressants: data preliminary. Ann. Intern. (Caracas), 16, 177-181.
- Gawarammana, I., Buckley, N.A., Mohamed, F., Naser, K., Jeganathan, K., Ariyananada, P.L., Wunnapuk, K., Dobbins, T.A., Tomenson, J.A., Wilks, M.F., Eddleston, M. and Dawson, A.H. (2018): High-dose immunosuppression to prevent death after paraquat self-poisoning - a randomised controlled trial. Clin. Toxicol. (Phila.), 56, 633-639.
- Gawarammana, I.B. and Buckley, N.A. (2011): Medical management of paraquat ingestion. Br. J. Clin. Pharmacol., 72, 745-757.
- Ge, W., Wang, H.L. and Sun, R.P. (2014): Clinical characteristics of paraquat poisoning in 22 Chinese children. Indian J. Pediatr., 81, 670-674.
- He, F., Xu, P., Zhang, J., Zhang, Q., Gu, S., Liu, Y. and Wang, J. (2015): Efficacy and safety of pulse immunosuppressive therapy with glucocorticoid and cyclophosphamide in patients with paraquat poisoning: A meta-analysis. Int. Immunopharmacol., 27, 1-7.
- Ho, K.M. and Tan, J.A. (2011): Use of L’Abbé and pooled calibration plots to assess the relationship between severity of illness and effectiveness in studies of corticosteroids for severe sepsis. Br. J. Anaesth., 106, 528-536.
- Hsieh, Y.W., Lin, J.L., Lee, S.Y., Weng, C.H., Yang, H.Y., Liu, S.H., Wang, I.K., Liang, C.C., Chang, C.T. and Yen, T.H. (2013): Paraquat poisoning in pediatric patients. Pediatr. Emerg. Care, 29, 487-491.
- Hsu, C.W., Lin, J.L., Lin-Tan, D.T., Chen, K.H., Yen, T.H., Wu, M.S. and Lin, S.C. (2012): Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS One, 7, e48397.
- Jian, X.D., Guo, G.R., Ruan, Y.J., Wang, Y.C., Ning, Q., Zhao, B., Gao, D.M., Li, P., Feng, F.R., Guo, J.R., Wang, X., Lin, D.W. and Sun, G. (2008): [Clinical study on treatment of acute paraquat poisoning]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 26, 549-552.
- Koo, J.R., Yoon, J.W., Han, S.J., Choi, M.J., Park, I.I., Lee, Y.K., Kim, S.G., Oh, J.E., Seo, J.W., Kim, H.J. and Noh, J.W. (2009): Rapid analysis of plasma paraquat using sodium dithionite as a predictor of outcome in acute paraquat poisoning. Am. J. Med. Sci., 338, 373-377.
- Li, L.R., Sydenham, E., Chaudhary, B., Beecher, D. and You, C. (2014): Glucocorticoid with cyclophosphamide for paraquat-induced lung fibrosis. Cochrane Database Syst. Rev., 8, CD008084.
- Li, Y., Wang, M., Gao, Y., Yang, W., Xu, Q., Eddleston, M., Li, L. and Yu, X. (2015): Abnormal pancreatic enzymes and their prognostic role after acute paraquat poisoning. Sci. Rep., 5, 17299.
- Lin, J.L., Leu, M.L., Liu, Y.C. and Chen, G.H. (1999): A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am. J. Respir. Crit. Care Med., 159, 357-360.
- Lin, J.L., Lin-Tan, D.T., Chen, K.H. and Huang, W.H. (2006): Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit. Care Med., 34, 368-373.
- Lin, J.L., Lin-Tan, D.T., Chen, K.H., Huang, W.H., Hsu, C.W., Hsu, H.H. and Yen, T.H. (2011): Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med., 37, 1006-1013.
- Lin, J.L., Wei, M.C. and Liu, Y.C. (1996): Pulse therapy with cyclophosphamide and methylprednisolone in patients with moderate to severe paraquat poisoning: a preliminary report. Thorax, 51, 661-663.
- Mantel, N. and Haenszel, W. (1959): Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst., 22, 719-748.
- Margulis, A.V., Pladevall, M., Riera-Guardia, N., Varas-Lorenzo, C., Hazell, L., Berkman, N.D., Viswanathan, M. and Perez-Gutthann, S. (2014): Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin. Epidemiol., 6, 359-368.
- Moon, J.M. and Chun, B.J. (2011): The efficacy of high doses of vitamin C in patients with paraquat poisoning. Hum. Exp. Toxicol., 30, 844-850.
- Panic, N., Leoncini, E., de Belvis, G., Ricciardi, W. and Boccia, S. (2013): Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One, 8, e83138.
- Perriëns, J.H., Benimadho, S., Kiauw, I.L., Wisse, J. and Chee, H. (1992): High-dose cyclophosphamide and dexamethasone in paraquat poisoning: a prospective study. Hum. Exp. Toxicol., 11, 129-134.
- Scherrmann, J.M., Houze, P., Bismuth, C. and Bourdon, R. (1987): Prognostic value of plasma and urine paraquat concentration. Hum. Toxicol., 6, 91-93.
- Sun, I.O. and Lee, K.Y. (2013): Cyclophosphamide dose: how much is needed to win the war against paraquat poisoning? Korean J. Intern. Med. (Korean. Assoc. Intern. Med.), 28, 410-412.
- Tian, Y.P., Shi, H.W. and Meng, N. (2010): [Clinical analysis of five cases of necrosis of femoral head after acute paraquat poisoning]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 28, 790-791.
- Vieira, R.J., Zambrone, F.A., Madureira, P.R. and Bucaretchi, F. (1997): Treatment of paraquat poisoning using cyclophosphamide and dexamethasone. J. Toxicol. Clin. Toxicol., 35, 515-516.
- Wang, H.R., Pan, J., Shang, A.D. and Lu, Y.Q. (2017): Time-dependent haemoperfusion after acute paraquat poisoning. Sci. Rep., 7, 2239.
- Wang, J.R., Kan, B.T. and Jian, X.D. (2013): [Hormonotherapy for treating femoral head necrosis induced by paraquat poisoning: a report of 2 cases]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 31, 394.
- Wang, W.S., Ma, Z.X., Lu, Q.L. and Zhang, X. (2008): Clinical study of high dose methylprednisolone combined with cyclophosphamide to improve pulmonary injury after paraquat poisoning. Zhonghua Jizhen Yixue Zazhi, 17, 757-759.
- Welch, V., Petticrew, M., Petkovic, J., Moher, D., Waters, E., White, H. and Tugwell, P.; PRISMA-Equity Bellagio group. (2016): Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. J. Clin. Epidemiol., 70, 68-89.
- Wu, W.P., Lai, M.N., Lin, C.H., Li, Y.F., Lin, C.Y. and Wu, M.J. (2014): Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS One, 9, e87568.
- Xu, J.J., Zhen, J.T., Tang, L. and Lin, Q.M. (2017): Intravenous injection of Xuebijing attenuates acute kidney injury in rats with paraquat intoxication. World J. Emerg. Med., 8, 61-64.
- Xu, L., Hu, P.B., Lv, Y. and Qiu, J.Q. (2012): Effect of cyclophosphamide combined with cyclosporine A on the prognosis of acute paraquat poisoning. Chin. J. Pract. Med., 39, 44-46.
- Yoshii, A., Plaut, D.A., McGraw, K.A., Anderson, M.J. and Wellik, K.E. (2009): Analysis of the reporting of search strategies in Cochrane systematic reviews. J. Med. Libr. Assoc., 97, 21-29.